Efficient conversion of biomass-derived furfuryl alcohol to levulinate esters over commercial α-Fe2O3†
Abstract
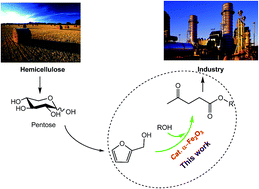
An efficient process for the production of levulinate esters from biomass-derived furfuryl alcohol in liquid alcohol over commercial α-Fe2O3 was firstly investigated. Among the catalysts we tested, α-Fe2O3, a cheap, commercially available and environmentally benign catalyst, exhibited a remarkable catalytic performance for the transformation and gives levulinate esters in good yield compared to the previous studies. The corresponding esters such as methyl levulinate, ethyl levulinate and butyl levulinate were obtained in high yields under optimized reaction conditions. Several influence factors for the formation of levulinate esters were also discussed. A plausible reaction mechanism for the formation of levulinate ester from furfuryl alcohol was proposed. From the viewpoint of practice and economy, the present study provides a potential application for the efficient synthesis of fine chemicals from biomass-derived compounds over cheap, commercially available and environmentally benign catalysts.

 Please wait while we load your content...
Please wait while we load your content...