Azole-based compounds as antiamoebic agents: a perspective using theoretical calculations†
Abstract
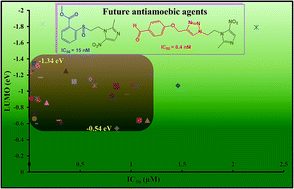
Diseases caused by protozoal organisms are responsible for significant mortality and morbidity worldwide. Amoebiasis caused by Entamoeba histolytica is an example of such diseases. In the quest for safe and effective antiamoebic agents, several heterocyclic moieties have been reported, out of which members of the azole family (dioxazole, pyrazoline, tetrazole, triazole and thiazolidinone derivatives) have attracted wide attention. This class of heterocyclic compounds have emerged as potential chemotherapeutic agents exhibiting promising antiamoebic activity with a non-cytotoxic nature. In the present article, some important breakthroughs in this area have been discussed. To get an insight at the supra-molecular level, computational studies like Lipinski's and DFT studies were carried out. Potent activity, chemical potential and hardness of the active compounds based on theoretical calculations were explained. The DFT study indicated that the LUMO energy level should lie between −1.34 and −0.54 eV to show high activity. We also observed that the LUMO level was mainly distributed over the 2-methyl 5-nitro imidazole ring in most of the active compounds.

 Please wait while we load your content...
Please wait while we load your content...