Changes in the SNAr reaction mechanism brought about by preferential solvation†
Abstract
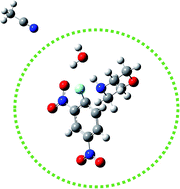
We herein report an experimental and theoretical study on preferential solvation effects for the reactions of 1-fluoro and 1-chloro-2,4-dinitrobenzene towards morpholine in acetonitrile, water and mixtures of them of varying compositions. A detailed kinetic study opens the possibility of analyzing preferential solvation and reaction rates. The kinetic study was complemented with an exploration of the potential energy surface in order to analyze the nature of the molecular interactions. For the fluorine derivative, this analysis reveals that the solvation of the TS in the mode TS1F-water/MeCN clearly outweighs the solvation of TS1F-MeCN/water, thereby suggesting that there is preferential solvation in favor of the aqueous phase.

 Please wait while we load your content...
Please wait while we load your content...