Copper(ii) acetate catalysed ring-opening cross-coupling of cyclopropanols with sulfonyl azides†
Abstract
A copper(II) acetate catalyzed ring-opening cross-coupling of cyclopropanol with sulfonyl azide has been developed. By this method, various β-amino ketones have been made efficiently in medium to high yields and venerable functional groups such as benzylic C–H, alkyl and aryl bromides, alkyl sulfonate, silyl ether and alkene are compatible with these reaction conditions. Control experiments have precluded the involvement of both radical and simple copper nitrene intermediates and a possible mechanism featuring key steps of ring-opening metalation and alkyl group migratory insertion into copper nitrene has been proposed.
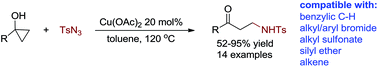

 Please wait while we load your content...
Please wait while we load your content...