Anion-exchange reaction synthesized CoNi2S4 nanowires for superior electrochemical performances†
Abstract
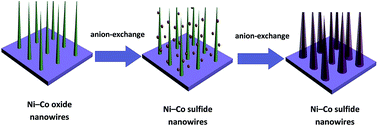
In this paper, we report CoNi2S4 nanowire arrays (NWAs) on 3D nickel foams based on an anion-exchange reaction which involved the pseudo Kirkendall effect. Due to the low electronegativity of sulfur, CoNi2S4 NWAs exhibit higher conductivity compared with Ni–Co oxide NWAs when used as active materials in supercapacitors. The electrochemistry tests show that these self-supported electrodes are able to deliver ultrahigh specific capacitance (1250 F g−1 at a current density of 5 mA cm−2), together with a considerable areal capacitance (2.5 F cm−2 at a current density of 5 mA cm−2), Furthermore, a capacitance retention of 72% after 5000 charge–discharge cycles at 5 mA cm−2 is obtained, indicating the excellent cycling stability of the CoNi2S4 NWA/nickel foam electrode. The superior electrochemistry capacity demonstrates that CoNi2S4 NWAs are promising electrode materials for supercapacitor applications.

 Please wait while we load your content...
Please wait while we load your content...