Cyclometalated iridium(iii) complexes of (aryl)ethenyl functionalized 2,2′-bipyridine: synthesis, photophysical properties and trans–cis isomerization behavior†
Abstract
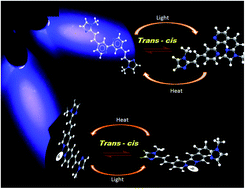
The syntheses, photophysical studies and photoinduced behavior of 4,4′-(aryl)ethenyl functionalized 2,2′-bipyridyls (L1, L2) and cyclometalated heteroleptic iridium(III) complexes (1, 2) with L1–L2 as ancillary ligands have been investigated in detail. The explicit characterization by time dependent 1H and 2D NMR, and time dependent electronic spectra supports an expected isomerization of L1 and L2 from trans–trans configuration to trans–cis and cis–cis configurations on exposure to 366 nm UV-vis light. Interestingly, the isomerization is restricted to only trans–cis configuration from the existing trans–trans form in the ligated L1 and L2 of complexes 1 and 2. The X-ray structure elucidation shows that the spatial arrangements of the (aryl)ethenyl moiety of L2 in complex 2, change with light exposure. The quantum chemical calculations by combined DFT-TDDFT give insight into the observed photophysical data. Furthermore, the rotational barriers of the isomerization of L1 and L2 were studied with variable temperature dependent 1H NMR.

 Please wait while we load your content...
Please wait while we load your content...