Penchinones A–D, two pairs of cis-trans isomers with rearranged neolignane carbon skeletons from Penthorum chinense†
Abstract
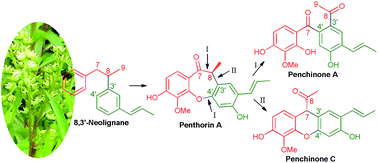
Penchinones A–D (1–4), two pairs of novel cis-trans lignan isomers with rearranged neolignane carbon skeletons, together with six known flavonoids (5–10), were isolated from the ethyl acetate-soluble portion of a hepatoprotective water decoction of Penthorum chinense. Their structures were determined by extensive spectroscopic data analysis, ECD calculation, and single-crystal X-ray diffraction. Penchinones C and D (3 and 4) featured an unprecedented 7,3′-neolignane carbon skeleton. Penchinone A (1) exhibited protective activity against acetaminophen-induced damage to the HL7702 hepatocyte and selective cytotoxicity against the human cancer ovarian cell line, Hey, in vitro.

 Please wait while we load your content...
Please wait while we load your content...