Efficient and selective extraction and determination of ultra trace amounts of Hg2+ using solid phase extraction combined with ion pair based surfactant-assisted dispersive liquid–liquid microextraction
Abstract
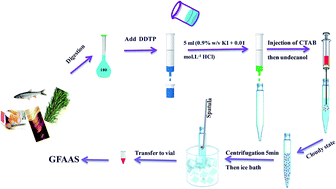
Solid phase extraction (SPE) was coupled with ion pair based surfactant-assisted dispersive liquid–liquid microextraction based on the solidification of the floating organic drop (IP-SA-DLLME-SFO) method, followed by graphite furnace atomic absorption spectrometry (GFAAS). At first, DDTP as a chelating agent was added to the sample solution of mercury ions and gently shaken for a few minutes. After complexation of mercury ions with diethyl dithiophosphate (DDTP), the sample was loaded on a solid phase extraction cartridge. The retained Hg2+ ions were then stripped from the cartridge with 5 mL of eluent (0.01 mol L−1 HCl and 1% w/v KI) in the form of HgI42− and then subjected to IP-SA-DLLME. In the proposed approach, cetyltrimethyl ammonium bromide (CTAB) was used as the emulsifier and ion pairing agent, and 1-undecanol was selected as the extraction solvent. In IP-SA-DLLME, a cationic surfactant was used in the extraction process. It has two fundamental functions: (1) the formation of an emulsified phase and (2) the ion pair formation with HgI42−, making it extractable into the organic phase. Variables affecting the performance of both steps were thoroughly investigated. A central composite chemometrics design was used for multivariate optimization of the effects of four different parameters influencing the extraction efficiency of IP-SA-DLLME. Response surface methodology (RSM) was applied to investigate the individual effects and possible interactions between the most effective variables, including the volume of the extraction solvent, salt addition and the concentration of KI and CTAB on the enrichment factor. The analytical characteristics of the method were determined. The calibration graph was linear in the rage of 0.02–20 μg L−1 with a detection limit of 0.009 μg L−1. The relative standard deviation for seven replicate measurements of 0.1 μg L−1 of mercury was 4.8%. The accuracy of the proposed method was evaluated by analyzing the fish homogenate IAEA-407 certified reference material. This procedure was also used as a novel extraction method for mercury speciation.

 Please wait while we load your content...
Please wait while we load your content...