Enhancing the effect of bisulfite on sequestration of selenite by zerovalent iron†
Abstract
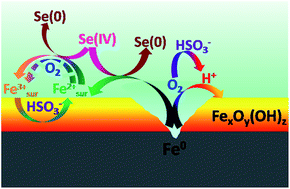
The enhancing effect of bisulfite (HSO3−) on the kinetics of Se(IV) sequestration by zerovalent iron (ZVI) was systematically investigated as a function of headspace volume, HSO3− concentration and initial pH (pHini). To exclude the role of HSO3− as an electrolyte, the kinetics of Se(IV) removal by ZVI with the presence of SO42− was determined as a control. With increasing headspace volume from 0 to 2.0 mL, the rate of Se(IV) removal by ZVI experienced a considerable enhancement whereas the further increase in the headspace volume resulted in a drop in Se(IV) removal rate. Se(IV) was always removed by ZVI with a higher rate in the presence of HSO3− than that in the presence of SO42− at various headspace volumes, which was mainly ascribed to the release of H+ and the depletion of O2 from the oxidation of HSO3− (i.e., 2HSO3− + O2 → 2SO42− + 2H+). Furthermore, HSO3− accelerated the reduction of ferric oxides and hydroxides to a Fe(II)-containing solid intermediate, which was beneficial to the reductive removal of Se(IV). The SEM, Fe K-edge XAFS and Se XANES analysis for Se(IV)-treated ZVI samples confirmed that HSO3− facilitated the transformation of ZVI to iron(oxyhydr)oxides (e.g., magnetite and lepidocrocite) and the reduction of Se(IV) to Se(0) compared to SO42−. The enhancing effect of HSO3− on Se(IV) sequestration varied with the concentration of HSO3− and initial pH, with the greatest effect achieved at 2.0 mM of Se(IV) and pHini 5.0. Since bisulfite is inexpensive and its final product is sulfate, a common anion existing in water, taking advantage of bisulfite to enhance the ZVI's reactivity under limited oxygenated conditions is a promising method.

 Please wait while we load your content...
Please wait while we load your content...