Photodegradation of Rhodamine B in α-FeOOH/oxalate under light irradiation
Abstract
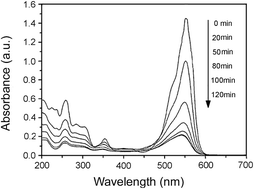
High- and low-crystalline goethite (α-FeOOH) were obtained via air oxidation of Fe(OH)2. Rhodamine B (RhB) was selected as a model pollutant, and the photocatalytic activity of the α-FeOOH/oxalate system under light irradiation was evaluated. The crystal structure, morphology, and specific surface area of the prepared α-FeOOH samples were determined by X-ray diffraction (XRD), transmission electron microscopy (TEM), and Brunauer–Emmett–Teller (BET), respectively. The adsorption behavior of α-FeOOH was determined using the Langmuir model. The effects of initial pH value, initial concentration of oxalic acid, and light intensity on RhB photodegradation were investigated in the α-FeOOH/oxalate suspension. In contrast to high-crystalline α-FeOOH, low-crystalline α-FeOOH with a high specific surface shows higher adsorption and photocatalytic activity toward RhB photodegradation by a photo-Fenton-like reaction. A possible mechanism for RhB degradation was also suggested.

 Please wait while we load your content...
Please wait while we load your content...