Four hybrid compounds based on a new type of molybdates and a flexible tripodal ligand: synthesis, structures, photochemical and electrochemical properties†
Abstract
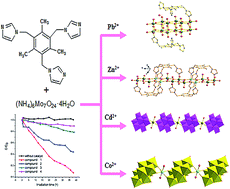
The hydrothermal reaction of a mixture of (NH4)6Mo7O24·4H2O, a flexible tripodal ligand 1,3,5-tris-(imidazol-1-ylmethyl)-2,4,6-trimethylbenzene (TITMB) and metal salts at different temperatures resulted in four novel compounds, namely [H2TITMB]2[Mo12O38]·4H2O (1), [Zn(Mo2O7)(TITMB)] (2), [H3TITMB]2[Cd(β-Mo8O26)(H2O)2(α-Mo8O26)]·4H2O (3) and [H3TITMB]2[Co(β-Mo8O26)(H2O)2(α-Mo8O26)]·4.5H2O (4). The structures of compounds 1, 2, 3 and 4 were explored with IR spectroscopy, thermal gravimetric analysis (TGA), PXRD, UV-vis diffuse reflectance spectra and single-crystal X-ray diffraction in the solid state. In 1 the new type of isopolymolybdate unit [Mo12O38]4− shared ten distorted {MoO6} octahedra and two distorted {MoO5} tetragonal pyramid with four kinds of O atoms. And the unprecedented bimetallic oxide chain [Zn(Mo2O7)]n in compound 2 consisted of trigonal bipyramids and octahedra through sharing edges. Both compounds 3 and 4 are 3-D octamolybdate-based inorganic–organic hybrids consisting of the polyacid [α-Mo8O26]4− anions and novel infinite chains of [β-Mo8O26]4− anions covalently linked through [Cd(H2O)2]2+ groups or [Co(H2O)2]2+ groups. In addition, the electrochemical behavior and photocatalytic activities of the title compounds were also investigated.

 Please wait while we load your content...
Please wait while we load your content...