Computational investigation of the influence of tetrahedral oxoanions (sulphate, selenate and chromate) on the stability of calcium carbonate polymorphs†
Abstract
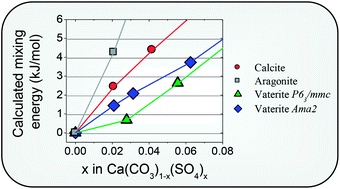
The incorporation of tetrahedral AO42− groups (A = S, Cr, Se) in CaCO3 polymorphs (calcite, aragonite and vaterite) is investigated from first principles calculations at the Density Functional Theory (DFT) level. We found that the less dense and softer vaterite crystal structure has greater capability to distort accommodating tetrahedral ions. The calculated mixing enthalpies at 0 K of the Ca(CO3)1−x(AO4)x (A = S, Se, Cr) vaterite and calcite polymorphs are below 3 kJ mol−1 when x < 0.05, confirming that the incorporation of small concentrations of tetrahedral groups is thermodynamically feasible in these polymorphs at moderate temperatures. Calcite is identified as the most stable polymorph at any investigated dopant concentration (0 < x < 0.25). Although our results do not predict stability crossovers resulting from AO42− group incorporation into CaCO3 polymorphs, they strongly support a reduction of the driving force for the transformation of AO4-bearing vaterite into the thermodynamically stable calcite.

 Please wait while we load your content...
Please wait while we load your content...