Growth of nano Ag@AgCl on (111) facets of Cu2O microcrystals with an enhanced photocatalytic activity
Abstract
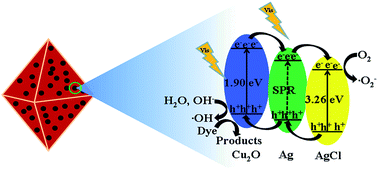
Single crystalline Cu2O nanoparticles were synthesized under mild conditions. A Ag@AgCl/Cu2O photocatalyst was prepared by directly growing Ag@AgCl nanoparticles (NPs) on (111) facets of octahedral Cu2O via a facile precipitation in situ photoreduction method. The results indicate that Ag@AgCl nanoparticles have a narrow size distribution ranging from 10 to 50 nm and are uniformly distributed on the surface of Cu2O nanoparticles. The surface area of the composite reached up to 19.736 m2 g−1. The photocatalytic performance of the Ag@AgCl/Cu2O composite for the degradation of methylene blue (MB) was evaluated under visible light irradiation. The Ag@AgCl/Cu2O composite with 30 wt% Ag@AgCl showed the highest photocatalytic activity, degrading 93.61% MB after 2 h irradiation. The high photocatalytic activity of the Ag@AgCl/Cu2O composite can be attributed to its high surface area, the crystal effect of Cu2O and the surface plasmon resonance of the Ag NPs. In addition, the Ag@AgCl/Cu2O composite can be used as a photocatalyst for the degradation of phenol. Based on these experimental results, a photocatalytic mechanism for the degradation of hazardous chemical effluents over Ag@AgCl/Cu2O photocatalysts was proposed. The free radicals and holes act as the main reactive species during the degradation.

 Please wait while we load your content...
Please wait while we load your content...