(S)-Naproxen based novel chiral reagent for C–N bond formation: enantioseparation of some β-blockers, determination of absolute configuration and elution order of diastereomers†
Abstract
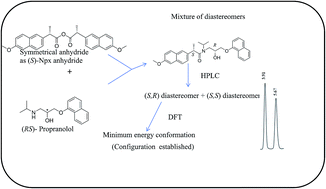
A new chiral derivatizing reagent has been synthesized from (S)-(+)-naproxen and was used for C–N bond formation to prepare diastereomeric amides of (RS)-propranolol, (RS)-atenolol, (RS)-carvedilol and (RS)-metoprolol. Derivatization reactions were done at room temperature (30 °C for 30 min) under stirring conditions as well as under microwave irradiation (MWI). Separation of diastereomers was achieved by open column chromatography. 1H NMR spectra of the isolated and purified diastereomers were recorded to establish the configurations of the first and second eluting diastereomers (and thus the elution order) and to compare the chromatographic separation characteristics when the diastereomeric mixture was separated using achiral RP-HPLC (using C18 column and a binary mixture of MeCN and triethyl ammonium phosphate buffer of pH 3.5 (60 : 40 v/v) as mobile phase at a flow rate of 1 mL min−1 and UV detection at 230 nm). No racemization was observed throughout the study. Test samples of β-blockers were isolated from commercial tablets and then were purified and characterized to be used as racemic standard. The conditions for derivatization and separation were optimized. Lowest energy optimized structures of the two diastereomers were developed using the Gaussian 09 Rev. A.02 program and hybrid density functional B3LYP with 6-31G basis set which supplemented 1H NMR interpretations and confirmed three dimensional geometry of the diastereomers. Separation method was validated as per ICH guidelines. The limit of detection and limit of quantification for each isomer were 0.4 ng mL−1 and 1.2 ng mL−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...