Captopril as a nucleophile for ester cleavage. Formation of the thiolester S-benzoylcaptopril†
Abstract
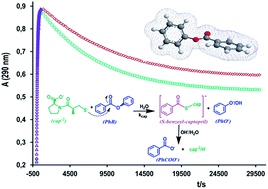
The rates of both acyl transfer from phenylacetate (PhA) or acetylsalicylic acid (ASA), and benzyl transfer from phenylbenzoate (PhB) to the hydroxide ion and to the thiol anion of captopril (cap) or cysteine (cys) have been determined in an aqueous basic solution. The rates of ester cleavage by OH− are faster than that promoted by the thiol anion of either cap or cys. In every case, the pseudo-first order rate constant shows linear dependence on either [OH−] or [thiol]. Nevertheless, for the reaction between PhB and cap, the absorbance–time profiles obey consecutive reactions, where the two reaction paths were attributed to the rapid formation of S-benzoylcaptopril that subsequently decomposes in a slower reaction step to form phenolate and benzoate, as stable products. The S-benzoylcaptopril decomposition is accelerated in an alkaline medium and is practically suppressed in a carbonate buffer of pH 10. In addition, the presence of cationic micelles at concentration values close the cmc, not only accelerates the formation of the thiolester, but also decreases its decomposition. The second-order rate constant for the reaction between the three nucleophiles and PhA or ASA correlates quite well with the basicity (pKa) of the nucleophile, a fact that suggests the same reaction mechanism. In contrast, in the correlation for PhB, the data corresponding to the reaction with cap deviates significantly from the linear plot, which evidences a different reaction scheme.

 Please wait while we load your content...
Please wait while we load your content...