One-step preparation of nickel sulfide/nickel hydroxide films for electrocatalytic hydrogen generation from water†
Abstract
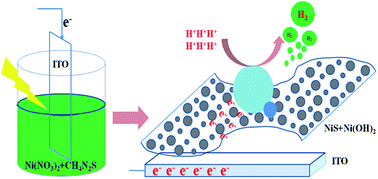
Electrocatalytic reduction of water to molecular hydrogen via the hydrogen evolution reaction (HER) may provide a sustainable energy supply for the future, but its commercial application is hampered by the use of precious metals and high preparation cost. In this work, we report that Ni-S films prepared by one-step photo-assisted potentiodynamic deposition are active HER catalysts in aqueous media. Notably, the Ni-S films showed efficient and robust electrocatalytic activity for the HER in water over a wide pH range. A long-term bulk electrolysis of the Ni-S film exhibited steady current over 22 h without deactivation, demonstrating its superior stability in neutral water. The facile preparation of this Ni-S film, along with its low overpotential, high activity, and long-term aqueous stability, offer promising features for potential use in H2 evolution applications. A set of characterization techniques, including field emission scanning electron microscopy (FE-SEM), powder X-ray diffraction patterns (XRD) and X-ray photoelectron spectroscopy (XPS), were conducted to detect the composition and structure of the Ni-S film, revealing that its major component is NiS/Ni(OH)2.

 Please wait while we load your content...
Please wait while we load your content...