Thermal decomposition mechanism of Co–ANPyO/CNTs nanocomposites and their application to the thermal decomposition of ammonium perchlorate
Abstract
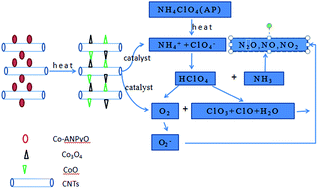
A chemical precipitation method was used to prepare cobalt complexes of 2,6-diamino-3,5-dinitropyridine-1-oxide/carbon nanotube (Co–ANPyO/CNTs) nanocomposites. The structure and thermal analyses indicate that Co–ANPyO nanoparticles are well dispersed on the surface of CNTs with an average particle size of about 10 nm, the content of Co–ANPyO nanoparticles in nanocomposites is about 73.4 wt%. The thermal decomposition mechanism of Co–ANPyO and Co–ANPyO/CNTs nanocomposites were predicted based on thermogravimetry-differential scanning calorimetry (TG-DSC) and thermolysis in situ rapid-scan FTIR (RSFTIR) results. The thermal decomposition of Co–ANPyO and Co–ANPyO/CNTs nanocomposites contains two exothermic processes in the temperature range of 25–490 °C. The first exothermic process for Co–ANPyO/CNTs nanocomposites shifts towards lower temperatures compared to that of Co–ANPyO. The main products of the final residues for Co–ANPyO and Co–ANPyO/CNTs nanocomposites at 490 °C are Co3O4 and CoO, respectively. The catalytic performance of Co–ANPyO and Co–ANPyO/CNTs nanocomposites on thermal decomposition of ammonium perchlorate (AP) was investigated by TG-derivative thermogravimetry (DTG), DSC, non-isothermal kinetic and α–T kinetic curves analyses. The possible catalytic mechanism was also discussed and proposed. During the thermal decomposition process of AP with Co–ANPyO/CNTs nanocomposites, Co–ANPyO/CNTs nanocomposites might decompose and form Co3O4/CNTs and CoO/CNTs nanocomposites as high activity catalysts, which could accelerate the thermal decomposition of AP. Thus, Co–ANPyO/CNTs nanocomposites not only lower the decomposition temperature and activation energy, but also enhance the total heat of AP, which could not be achieved by the CNTs and Co–ANPyO alone. The way of preparing Co–ANPyO/CNTs nanocomposites presented in this work can be expanded to other energetic additives/CNTs nanocomposites used for AP and AP based propellants.

 Please wait while we load your content...
Please wait while we load your content...