A polymerized ionic liquid functionalized cathode catalyst support for a proton exchange membrane CO2 conversion cell†
Abstract
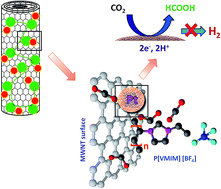
This study aims at the efficient conversion of CO2 to formic acid using a proton exchange membrane cell by selective functionalization of a cathode catalyst support. We chose polymerized ionic liquid (PIL) as the surface functional moiety, since CO2 has good solubility in it. A multiwalled carbon nanotube (MWNTs) surface was functionalized with PIL and used as a cathode catalyst support. This novel catalyst support shows extremely good affinity towards CO2 and facilitates better dispersion of catalyst nanoparticles. Catalytic nanoparticles were decorated over the catalyst supports by a microwave assisted polyol reduction method, which gives better dispersion of finer particles on PIL functionalized MWNTs compared to pure MWNTs. The protonation of CO2 to formic acid has been studied in a polymer electrolyte membrane (PEM) CO2 conversion cell with synthesized catalysts. The cells were tested under continuous and discontinuous CO2 supply, where PIL functionalized MWNTs show a better formic acid formation rate than the pure support under identical experimental conditions, due to the improved interaction between the catalyst support and CO2 molecules.

 Please wait while we load your content...
Please wait while we load your content...