Effect of ionic composition on the partitioning of organic compounds in octanol–buffer systems†
Abstract
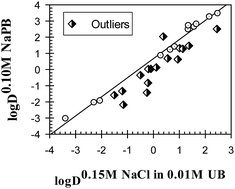
The distribution of 29 drug compounds was examined in octanol–buffer systems with eight different ionic compositions at pH 7.4. It was found that the ionic composition of the octanol–buffer system has a noticeable and unpredictable effect on the log D7.4 values for different compounds. It was established that the response of the compounds to different ionic environments, displayed as log D7.4 values, are linearly interrelated, which is similar to the different properties of compounds in organic solvent-free aqueous solutions reported previously [Ferreira et al., Phys. Chem. Chem. Phys. 2014, 16, 23347]. An analysis of the role of the different structural features of compounds examined in their ionic responsiveness showed that the molecular polarizability and polar surface area are the two most important features. This suggests that the relationships reported here and previously are based on the same physical principles that must be considered in any theoretical model of solute–water interactions in aqueous solutions containing salt additives.

 Please wait while we load your content...
Please wait while we load your content...