Studies on the electrochemical intercalation/de-intercalation mechanism of NiMn2O4 for high stable pseudocapacitor electrodes†
Abstract
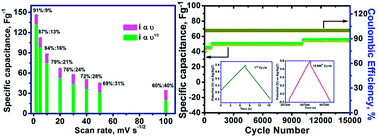
Sub-micron sized polyhedral shaped NiMn2O4 particles were successfully prepared by a glycine assisted solution combustion method. The phase purity and the presence of functional groups in NiMn2O4 were revealed through X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR), respectively. The formation of polyhedral shaped particles was inferred by field emission scanning electron microscopy (FE-SEM). The negative temperature coefficient of resistance (NTCR) behaviour of NiMn2O4 was observed using a solid state impedance analyser in the measured temperature range between 30 and 180 °C. Further, electrochemical studies revealed that NiMn2O4 stores the charge through intercalation rather than by a capacitive mechanism. The electrode stores 91% of the specific capacitance by intercalation and 9% by a capacitive mechanism. Also, NiMn2O4 possesses a specific capacitance of 202 F g−1 at 0.5 mA cm−2 in 1 M Na2SO4 electrolyte and exhibits excellent cyclic stability over 15 000 cycles. Similarly, the fabricated asymmetric device (FeVO4‖NiMn2O4) also delivers good specific capacitance (50 F g−1 at 1 mV s−1) and cyclic stability.

 Please wait while we load your content...
Please wait while we load your content...