Base catalysed domino and self-domino Michael–Aldol reactions: one-pot synthesis of dispirocyclopentaneoxindoles containing multiple chiral stereocenters†
Abstract
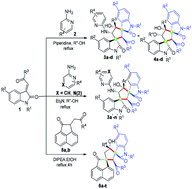
A one-pot method for the construction of three classes of densely functionalized dispirocyclopentaneoxindoles containing multiple chiral stereocenters is developed. Base promoted self-domino and domino reactions with and without the participation of solvent, alcohol, is accomplished. Piperidine catalyzed self-domino reaction involves the participation of the nucleophilic solvent, alcohol, resulting in complex dispiro compounds containing four and five diastereoisomeric centers. Triethylamine promotes the self-domino reaction between two molecules of 3-phenacylideneoxindoles with added nucleophiles, and not with the solvent nucleophile. Diisopropylethylamine facilitates the domino reaction between 3-phenacylidineoxindole and phenacylacenaphthylenone allowing the construction of novel dispirocyclopentaneoxindoles with high yields and diastereoselectivity. A plausible mechanism is tentatively proposed to account for the diastereoselectivity of the domino Michael–Aldol reaction.

 Please wait while we load your content...
Please wait while we load your content...