Polystyrene resin supported palladium(0) (Pd@PR) nanocomposite catalyzed synthesis of β-aryl and β,β-diaryl unsaturated scaffolds following tandem approaches†‡
Abstract
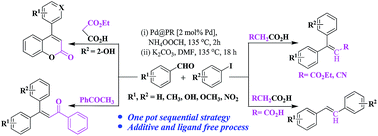
A one pot general tandem procedure is described for β-aryl and β,β-diaryl alkenes synthesis following an alternative to the classical approaches by using aryl aldehyde as one of the starting materials. The developed polystyrene resin supported palladium(0) (Pd@PR) nanocomposite has been applied in an unprecedented sequential condensation–decarboxylation–Heck (CDH) and condensation–Heck (CH) strategies to generate the substituted alkenes (C6(C6)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C, C6–C
C–C, C6–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C6, and C6(C6)C
C–C6, and C6(C6)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO–C6 units) under ligand, and additive free milder basic reaction conditions. The added momentous benefit over the classical methodologies is in terms of its multi-component approach to achieve the desired products without tedious step wise purifications.
C–CO–C6 units) under ligand, and additive free milder basic reaction conditions. The added momentous benefit over the classical methodologies is in terms of its multi-component approach to achieve the desired products without tedious step wise purifications.

 Please wait while we load your content...
Please wait while we load your content...