Glycal approach to the synthesis of macrolide (−)-A26771B†
Abstract
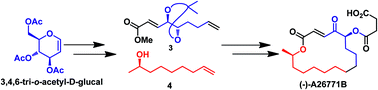
A convergent total synthesis of a 16-membered macrolactone natural product (−)-A26771B 1 starting from 3,4,6-tri-O-acetyl-D-glucal 7 is reported. The Ferrier rearrangement of acetylated glucal 7, cross metathesis between chiral fragments 3 and 4, Yamaguchi macrolactonization and selective oxidation of the allylic alcohol are the key features of the synthesis.

 Please wait while we load your content...
Please wait while we load your content...