New insights into the effects of NaCl and LiCl on the hydrogen storage behaviours of a 6LiBH4–Mg(AlH4)2 composite
Abstract
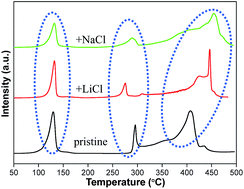
The effects of the NaCl and LiCl by-products generated during the synthesis of Mg(AlH4)2 on the hydrogen storage properties of a 6LiBH4–Mg(AlH4)2 composite are investigated and clarified for the first time. The results indicate that the presence of NaCl and LiCl changes the dehydrogenation/hydrogenation kinetics of the 6LiBH4–Mg(AlH4)2 composite in addition to producing a distinct reduction in the hydrogen capacity. For the NaCl-containing sample, the chemical composition is changed due to the metathesis reaction between NaCl and LiBH4 during ball milling, which converts the NaCl and LiBH4 to LiCl and NaBH4. However, for the LiCl-containing system, the kinetic barriers of the dehydrogenation reaction were changed by the presence of LiCl, which is responsible for the change in the dehydrogenation temperature. These findings elucidate the effects of NaCl and LiCl, which are produced during the synthesis of Mg(AlH4)2, on the hydrogen storage behaviours of the 6LiBH4–Mg(AlH4)2 composite.

 Please wait while we load your content...
Please wait while we load your content...