Exploring the mode of binding of the bioflavonoid kaempferol with B and protonated forms of DNA using spectroscopic and molecular docking studies†
Abstract
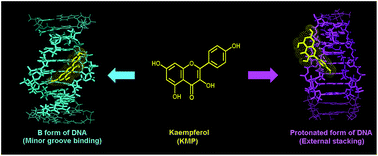
Protonation-induced conformational changes in natural DNAs under the conditions of low pH at low temperature, and low ionic strength have been studied using various spectroscopic techniques. At pH 3.4, 10 mM [Na+], and at 10 °C, natural DNAs adopt an unusual and stable conformation remarkably different from the canonical B-form conformation. The protonated conformation of naturally occurring calf thymus (CT) DNA has been characterized by UV-Vis absorption and circular dichroism (CD) studies. Binding interaction of kaempferol (KMP), a bioactive flavonoid, with B-form and the protonated form of CT DNA has been explored using various spectroscopic techniques. The determined binding constant, fluorescence quenching experiment, viscosity measurement, CD study, helix melting study and molecular docking simulation confirm the groove binding of KMP with B-form and external stacking interaction with the protonated form of CT DNA. The dual fluorescence of KMP resulting from the excited state intramolecular proton transfer is modified remarkably upon binding with the protonated form of DNA. This is the first report so far where a naturally occurring flavonoid has been shown to bind to the protonated form of DNA.

 Please wait while we load your content...
Please wait while we load your content...