Investigation into the reactivity of 16-electron complexes Cp#Co(S2C2B10H10) (Cp# = Cp, Cp*) towards methyl diazoacetate and toluenesulphonyl azide†
Abstract
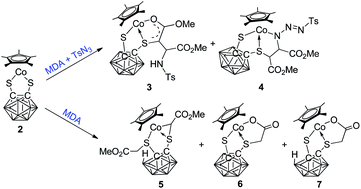
A three-component reaction of the 16-electron half-sandwich complex Cp*Co(S2C2B10H10) (2) with methyl diazoacetate (MDA) and toluenesulphonyl azide (TsN3) gave di-inserted products Cp*Co(S2C2B10H10)(C–CO2Me)(CHCO2Me)(NHTs) (3) and Cp*Co(S2C2B10H10)(CHCO2Me)(CHCO2Me)(N3Ts) (4), where MDA and TsN3 in a molar ratio of 2 : 1 were inserted into the Co–S bond to form a five-membered metallacyclic ring with different coordination modes. Its two-component reaction with MDA affording complexes Cp*Co(S2C2B9H9)(CH2CO2Me)(CHCO2Me) (5), Cp*Co(S2C2B10H10)(CH2CO2) (6) and Cp*Co(S2C2B9H9) (CH2CO2) (7) proved that the abovementioned three-component reaction is not a simple stepwise reaction. For another 16-electron half-sandwich complex CpCo(S2C2B10H10) (1), two stepwise reaction routes were designed to achieve its incorporation with MDA and TsN3. The reaction occurred via the alkylidene-bridged adduct CpCo(S2C2B10H10)(CHCO2Me) (8) or the imido-bridged adduct CpCo(S2C2B10H10)(NTs) (9), and complex CpCo(S2C2B10H10)(CHCO2Me)(NTs) (10) was isolated as the sole product in moderate yield. In each reaction route, one molecule of MDA and one molecule of TsN3 insert into the two Co–S bonds of complex 1, respectively, with the loss of N2. All the new complexes were characterized using NMR spectroscopy, mass spectrometry, IR spectroscopy, elemental analysis and X-ray structural analysis.

 Please wait while we load your content...
Please wait while we load your content...