Spiropyran-decorated light-responsive amphiphilic poly(α-hydroxy acids) micelles constructed via a CuAAC reaction†
Abstract
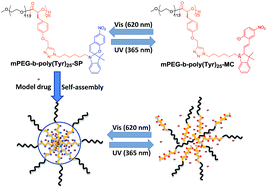
Light-responsive amphiphilic poly(α-hydroxy acids) mPEG-b-poly(Tyr)-SP was prepared by the introduction of a spiropyran chromophore into the side chain of poly(ethylene glycol)-modified poly(α-hydroxy acids) (mPEG-b-poly(Tyr)) via a copper-catalysed azide–alkyne cycloaddition (CuAAC) reaction. The resultant copolymer can self-assemble into spherical micelles with an average diameter of 222.7 nm and a critical micelle concentration of 0.0085 mg mL−1. The micelles showed reversible self-assembly and disassembly in aqueous solution under alternative UV and visible light irradiation. The model drug coumarin 102 was then encapsulated into the micelles successfully. Light-controlled release and re-encapsulation behaviours were demonstrated by fluorescence spectroscopy. MTT assay revealed that the mPEG-b-poly(Tyr)-SP micelles exhibited excellent cell compatibility. This study provides a convenient way to construct smart poly(α-hydroxy acids)-based nanocarriers for controlled release and re-encapsulation of hydrophobic drugs.

 Please wait while we load your content...
Please wait while we load your content...