2,4-Dinitrobenzenesulfonic acid in an efficient Brønsted acid-catalyzed controlled/living ring-opening polymerization of ε-caprolactone†
Abstract
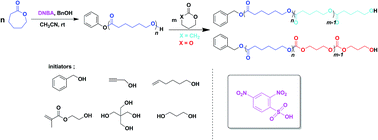
The ring-opening polymerization of ε-caprolactone (ε-CL) using benzyl alcohol (BnOH) as initiator and 2,4-dinitrobenzenesulfonic acid (DNBA) as catalyst in acetonitrile at room temperature with a [ε-CL]0/[BnOH]0/[DNBA]0 ratio of 40/1/1 has been investigated. The polymerization proceeded to obtain poly(ε-caprolactone) (PCL) with controlled molecular weights. In addition, 1H NMR, SEC, and MALDI-ToF MS measurements demonstrated the initiator residue at the polymer chain end. Furthermore, propargyl alcohol, 5-hexen-1-ol, 2-hydroxyethyl methacrylate, 1,3-propanediol, and pentaerythritol were used as functional initiators to successfully obtain end-functionalized and α,ω-dihydroxy telechelic polyesters. The block copolymerization of PCL and PVL or PTMC provided conditions to afford well-defined poly(ε-caprolactone)-block-poly(δ-valerolactone) (PCL-b-PVL) and poly(ε-caprolactone)-block-poly(trimethylene carbonate) (PCL-b-PTMC).


 Please wait while we load your content...
Please wait while we load your content...