The efficient enrichment of U(vi) by graphene oxide-supported chitosan
Abstract
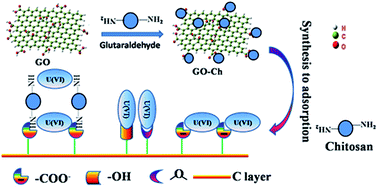
Graphene oxide-supported chitosan (GO-Ch) composites were synthesized using a covalent method for U(VI) adsorption and were characterized by scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), differential thermal analysis (DTA) and extended X-ray absorption fine structure (EXAFS). The characteristic results indicated that Ch was successfully grafted onto GO. The adsorption of U(VI) on GO-Ch was investigated under different environmental conditions. The adsorption kinetics showed that the adsorption of U(VI) on GO-Ch followed the pseudo-second-order equation. The maximum adsorption capacity of U(VI) on GO-Ch at pH 4.0 and T = 303 K calculated from the Langmuir model was 225.78 mg g−1. Thermodynamic parameters calculated from temperature-dependent adsorption isotherms suggested that U(VI) adsorption on GO-Ch was an endothermic and spontaneous process. The batch desorption indicated U(VI) cannot be completely desorbed from GO-Ch without intervention, suggesting the irreversible adsorption of U(VI) on GO-Ch. The analysis of FT-IR spectra suggested that the interaction mechanism of U(VI) on GO-Ch was mainly chemical adsorption by –NH2 and –COOH groups. According to EXAFS analysis, the peaks at ∼2.9 Å can be satisfactorily fitted by the U–C/N shell, revealing the formation of inner-sphere surface complexes. It is demonstrated that the GO-Ch nanocomposite can be a promising material for the preconcentration and solidification of U(VI) from large volumes of aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...