Multifunctional nanocomposite Fe3O4@SiO2–mPD/SP for selective removal of Pb(ii) and Cr(vi) from aqueous solutions
Abstract
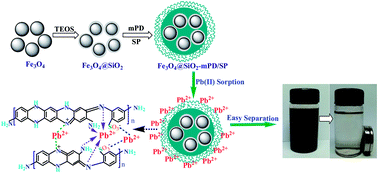
Silica-coated magnetite (Fe3O4@SiO2) nanoparticles functionalized with amino, imino and sulfonic groups (Fe3O4@SiO2–mPD/SP) were successfully synthesized via a facile chemical oxidative polymerization of m-phenylenediamine (mPD) and m-sulfophenylenediamine-4-sulfonic acid (SP) monomers, and utilized for selective removal of Pb(II) and Cr(VI) from aqueous solutions. It was revealed by the characterizations that the polymers formed on Fe3O4@SiO2 nanoparticles were the true copolymers with a mPD–SP unit, rather than a mixture of mPD and SP homopolymers. Fe3O4@SiO2–mPD/SP nanocomposites could be easily separated from aqueous solutions within 30 s. The maximum adsorption capacities of Pb(II) (83.23 mg g−1) and Cr(VI) (119.06 mg g−1) on Fe3O4@SiO2–mPD/SP nanocomposites were obtained at the mPD/SP molar ratios of 95 : 5 and 50 : 50, respectively. Moreover, satisfactory selective removal of Pb(II) and Cr(VI) from their mixtures with Cu(II) and Ni(II) ions were exhibited by the Fe3O4@SiO2–mPD/SP (95 : 5) and Fe3O4@SiO2–mPD/SP (50 : 50), respectively. The Pb(II) adsorption equilibrium was reached within 5 min by Fe3O4@SiO2–mPD/SP (95 : 5). The adsorption data of Pb(II) and Cr(VI) were both fitted well to the Freundlich isotherm and followed the pseudo-second-order kinetic model. The adsorption mechanism of Pb(II) and Cr(VI) on Fe3O4@SiO2–mPD/SP nanocomposites included five processes, namely: ion-exchange, complexation adsorption, reduction reaction, electrostatic attraction and physical adsorption. The enhanced adsorption performance of nanoparticle-based magnetic adsorbents for selective removal of heavy metal ions can be achieved with such a copolymerization strategy.

 Please wait while we load your content...
Please wait while we load your content...