Asymmetric Michael/hemiketalization of 5-hydroxy-2-methyl-4H-pyran-4-one to β,γ-unsaturated α-ketoesters catalyzed by a bifunctional rosin–indane amine thiourea catalyst†
Abstract
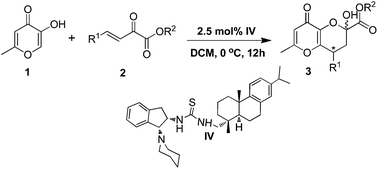
A highly efficient asymmetric cascade reaction namely Michael-hemiketalization of 5-hydroxy-2-methyl-4H-pyran-4one to β,γ-unsaturated α-ketoesters has been developed using a chiral bifunctional rosin–indane amine thiourea catalyst. The products are obtained in excellent yields with a high degree of enantiomeric excess (up to 99% ee), in a short reaction time with low catalyst loading of 2.5 mol%.

 Please wait while we load your content...
Please wait while we load your content...