Preparation of electrocatalysts by reduction of precursors with sodium citrate
Abstract
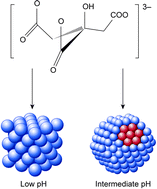
In this work synthesis of Pt/C catalysts by reduction of H2PtCl6 with sodium citrate has been investigated. The strong pH-dependence of citrate as a reducing and stabilizing agent has been explored, and an optimum pH range for production of well dispersed catalysts is proposed. To achieve stabilizing and reducing conditions, the presence of both citrate anions and protonated citrates are required. This is achieved in an intermediate pH range between pKa2 and pKa3 (4.76 and 6.4) of citric acid, where both C6H5O73− (denoted CA3−) and C6H7O6− (denoted H2CA−) are present. At pH 5.3–5.4 a catalyst with particles around 3 nm was thus successfully prepared. At high pH (∼12) the reduction of Pt is limited, whereas at low pH reduction is fast, but the stabilizing ability of the citrate in solution is poor resulting in large cubic Pt particles. CO-stripping voltammetry indicate that Pt(111) faces are the dominating crystal plane in the nanoparticles formed when citrate anions are used as stabilizing agent. This effect is presumably caused by the distance between oxygen groups in citrate correlating well with the Pt–Pt distance on (111) faces.

 Please wait while we load your content...
Please wait while we load your content...