Synthesis and electrochemical properties of graphene oxide/manganese oxide/polyaniline and its reduced composites
Abstract
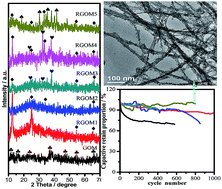
A graphene oxide/manganese oxide/polyaniline composite (GOM) was synthesized via a one-step method at room temperature, and its reduced graphene oxide/manganese oxide/polyaniline (RGOM) composites were prepared under different reaction conditions. The relationships between the synthesis approach, structure and electrochemical properties of the manganese oxide ternary composites were systematically investigated. The reaction temperature and the basic concentration played important roles in the reduction process. The possible reaction mechanisms of the ternary composites were proposed. The results show that high temperature under hydrothermal conditions can lead to a higher crystallinity degree, and promote the formation of the fiber-like nanostructure of the composites. Meanwhile, the higher concentration of NaOH promotes the reduction of MnO2 to other manganese oxides with a lower valence. The electrochemical characterization shows that, among those composites, the specific capacity of RGOM5 with a rough and sheathed nanostructure, obtained using 8 M NaOH at 120 °C via a hydrothermal method, can reach 344 F g−1 at a scan rate of 1 mV s−1, and the capacitive retention proportion remains nearly 100% after 6000 cycles, which presents a promising future for RGOM composites acting as low cost energy storage materials.

 Please wait while we load your content...
Please wait while we load your content...