Grindstone chemistry: a highly efficient and green method for synthesis of 3,4-dihydropyrimidin-2-(1H)-ones by l-tyrosine as an organocatalyst: a combined experimental and DFT study†
Abstract
A single step, mild, environmentally friendly green method has been developed for the synthesis of physiologically active 3,4-dihydropyrimidin-2-(1H)-ones employing L-tyrosine as catalyst under solvent-free conditions at room temperature via grinding. The procedure is efficient, time saving and gives high-yields. The structures and purity of these compounds were confirmed by FT-IR, NMR (1H and 13C) and HRMS spectral analysis. DFT calculations have been used to show the effectiveness of L-tyrosine as a suitable catalyst for the above reaction.
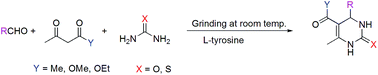

 Please wait while we load your content...
Please wait while we load your content...