Gel polymer electrolyte-based on PVDF/fluorinated amphiphilic copolymer blends for high performance lithium-ion batteries
Abstract
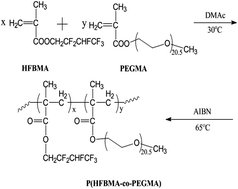
This paper describes the preparation and properties of PVDF/P(hexafluorobutyl methacrylate-co-poly(ethyleneglycol)methacrylate)(P(HFBMA-co-PEGMA)) blend gel polymer electrolyte (GPE) for high-performance lithium-ion batteries. The fluorinated amphiphilic copolymer, P(HFBMA-co-PEGMA), was synthesized by simple radical polymerization and then blended into poly(vinylidene fluoride) (PVDF) matrix via immersion phase inversion process. The composition, morphologies, liquid electrolyte uptake of the blend membranes and electrochemical properties of the corresponding GPEs were systematically investigated. It is found that the introduction of P(HFBMA-co-PEGMA) results in a slight increase in porosity, a reduction in crystallinity and better affinity with liquid electrolyte, which consequently lead to a substantial increase in liquid electrolyte uptake and ion conductivity. For the membrane with P(HFBMA-co-PEGMA)/PVDF mass ratio in 1.7/10, the liquid electrolyte uptake and ionic conductivity reach to 387% and 3.19 mS cm−1, respectively. In addition, the resulting GPE is electrochemically stable up to about 4.5 V (vs. Li+/Li).

 Please wait while we load your content...
Please wait while we load your content...