Benzylic-type couplings provide an important asymmetric entry to functionalized, non-racemic helicenes†
Abstract
Benzylic-type couplings are key reactions to make non-racemic helicenes. Their simplicity contributes to a short, efficient, and scalable asymmetric route to functionalized [7]helicenes. It widens the scope and uses of enantiopure helicenes, which are unequivocally needed for new chiroptical-electronic materials. A mechanistic proposal featuring an electrocyclization is based on experiments, X-ray crystallography and calculations.
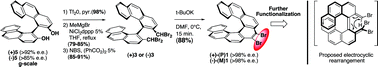

 Please wait while we load your content...
Please wait while we load your content...