Oxidant- and metal-free synthesis of 4(3H)-quinazolinones from 2-amino-N-methoxybenzamides and aldehydes via acid-promoted cyclocondensation and elimination†
Abstract
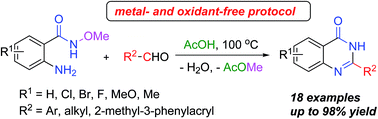
A series of biologically important 4(3H)-quinazolinones were readily synthesized in good to excellent yields from 2-amino-N-methoxybenzamides and aldehydes via a cascade reaction consisting of AcOH-promoted cyclocondensation and elimination. The current method sets itself apart from the conventional approach utilizing anthranilamide derivatives and aldehydes as building blocks, by its unique features, other than the high yields and one-pot procedure, including the absence of an oxidant, the elimination of a heavy-metal catalyst, and the formation of a non-toxic ester byproduct.

 Please wait while we load your content...
Please wait while we load your content...