Facile hydrothermal synthesis and luminescent properties of Eu-doped CaF2–YF3 alkaline-earth ternary fluoride microspheres†
Abstract
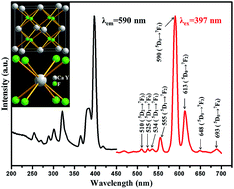
Large-scale CaF2–YF3 alkaline-earth ternary fluoride microspheres with diameters of about 2.5 μm were prepared by a facile hydrothermal method in the presence of disodium ethylenediamine tetraacetate (Na2H2L). The influences of several experimental parameters, such as reaction time, amount of Na2H2L, pH values, and fluoride source on the final products were investigated. The formation mechanism of the as-obtained microspheres was proposed on the basis of all these studies. It is also found that the addition amount of the Y3+ ions had an effect on the morphology of CaF2–YF3. The luminescence spectrum of Eu3+-doped CaF2–YF3 microspheres showed the strong characteristic dominant emission of the Eu3+ ions at 590 nm, indicating that the Eu3+ ions occupy a site of inversion symmetry in the CaF2–YF3 matrix.

 Please wait while we load your content...
Please wait while we load your content...