CH2Cl2 as reagent in the synthesis of methylene-bridged 3,3′-bis(oxazolidin-2-one) derivatives under ambient conditions†
Abstract
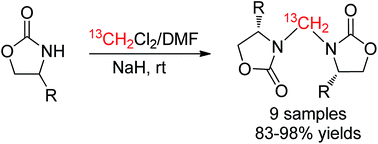
This paper describes a highly efficient and facile transformation of substituted oxazolidin-2-ones to methylene-bridged 3,3′-bis(oxazolidin-2-one) derivatives in a mixture solvent of CH2Cl2 (DCM) and N,N-dimethylformamide (DMF) in the presence of NaH at ambient temperature. Isotopic labeling experiments indicated that the C of the bridged methylene group was derived from CH2Cl2 instead of DMF and other unknown substances in the NaH reagent (60% dispersion in mineral oil).

 Please wait while we load your content...
Please wait while we load your content...