Synthesis, photophysical and electrochemical properties of chiral and achiral thiadiazolophanes†
Abstract
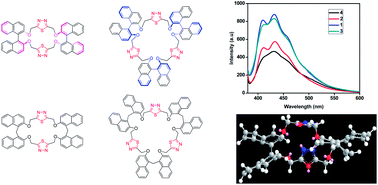
One pot synthesis of crown ether type chiral and achiral 2:2 oligomeric thiadiazolophane 1 and 3 and 3:3 oligomeric thiadiazolophane 2 and 4 with (S)-BINOL and a methylene bis-naphthyl spacer unit has been achieved under simple and mild conditions by O-alkylation methodology. Their photophysical and electrochemical properties revealed a higher degree of aggregation in 2:2 oligomer (1 and 3) and 3:3 oligomer cyclophanes 2 and 4 energy minimized calculations reveal that cyclophane with a bigger cavity has a lower heat of formation than cyclophane with a smaller cavity.

 Please wait while we load your content...
Please wait while we load your content...