A non-covalent complex based on catechol–benzoxazole moieties: electrochemical synthesis and characterization†
Abstract
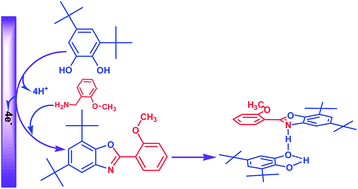
Electrochemical oxidation of 3,5-di-tert-butylcatechol has been studied in the presence of 2-methoxybenzylamine by means of cyclic voltammetry and controlled-potential coulometry. The results indicate that the electrogenerated 3,5-di-tert-butylcyclohexa-3,5-diene-1,2-dione participates in a cyclization reaction with 2-methoxybenzylamine and converts to the corresponding benzoxazole (4). Afterward by a non-covalent interaction, a proton transfer complex is formed between benzoxazole 4 and 3,5-di-tert-butylcatechol.

 Please wait while we load your content...
Please wait while we load your content...