Bimetallic Schiff-base aluminum complexes based on pentaerythrityl tetramine and their stereoselective polymerization of racemic lactide†
Abstract
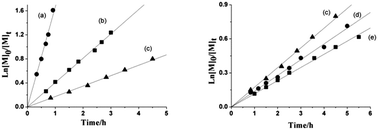
A series of Schiff base aluminum(III) complexes with bimetallic active centers are synthesized. Their catalytic properties in the solution polymerization of racemic lactide (rac-LA) are examined. The modifications in the auxiliary ligand exhibited a dramatic influence on the catalytic performance. Among these complexes, 3a has the highest stereoselectivity (Pm = 0.91) owing to the bulky tert-butyl groups on the salicylaldehyde. Kinetic studies indicate that the polymerizations are both first-ordered with respect to the monomer and catalyst. Other factors that influence the polymerization such as the polymerization time and the temperature, as well as the monomer concentration, are discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...