The mechanism for production of abiogenic formate from CO2 and lactate from glycerine: uncatalyzed transfer hydrogenation of CO2 with glycerine under alkaline hydrothermal conditions†
Abstract
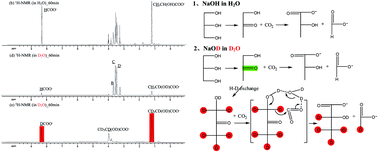
Recently, formate of possible abiogenic origin has been reported from uncatalyzed transfer hydrogenation of CO2 with glycerine under alkaline hydrothermal conditions. However, little is known about the mechanism for production of abiogenic formate from CO2 and lactate from glycerine during such processes. Herein, we investigated the formation of abiogenic formate from CO2, D2O solvent effect, reactor materials effect and H2O molecule catalysis for uncatalyzed transfer hydrogenation of CO2 with glycerine under alkaline hydrothermal conditions, and then have proposed and proved the potential reaction mechanism. The present work should help facilitate studies on industrial applications of CO2 reduction using abundant alcohol compounds as reducing materials rather than hydrogen, and the development of renewable high-valued chemicals from alternative biomass derivatives and the primary greenhouse gas to fossil fuel.

 Please wait while we load your content...
Please wait while we load your content...