Fabrication of Ni–Fe2O3 magnetic nanorods and application to the detection of uric acid†
Abstract
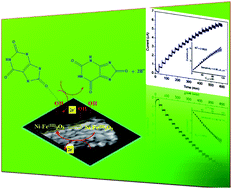
Doping of Ni into Fe2O3 lattices has been achieved by co-precipitation followed by thermal decomposition method. The structural, morphological, and magnetic properties of the fabricated samples were investigated by X-ray diffraction (XRD) analysis, X-ray photoelectron spectroscopy (XPS), Fourier transformed infrared (FT-IR) spectroscopy, UV-visible absorption (UV-vis) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and vibrating sample magnetometry (VSM). The results reveal that the Ni is well doped within the lattices of Fe2O3. The Ni dopant suppresses the formation of more stable α-Fe2O3 at higher calcination temperature. Further, the Ni-doped Fe2O3 nanoparticles were used to fabricate an electrochemical sensor (Ni–Fe2O3/GCE) for the detection of uric acid (UA) in biological conditions by cyclic voltammetry (CV) and chronoamperometry (CA). It was found that 5%Ni–Fe2O3/GCE exhibits best response towards UA with less positive potential and larger current response. Furthermore, the sensor gives good linear current response in the concentration range of 6.6 to 112.4 μM with the higher sensitivity of 0.849 μA (μM cm2)−1. Such fabricated sensors are appropriate for newly emerging non-enzymatic electrochemical nanobiosensors.

 Please wait while we load your content...
Please wait while we load your content...