Common precursor strategy for the synthesis of bestatin, amprenavir intermediate and syn-4-hydroxy-5-phenyl-γ-lactam†
Abstract
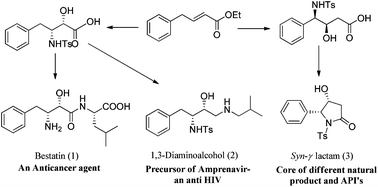
A common precursor strategy for the synthesis of bestatin hydrochloride, an anticancer agent, 1,3-diaminoalcohol, an amprenavir intermediate, and a syn-4-hydroxy-5-phenyl-γ-lactam intermediate of various bioactive molecules using an α,β-unsaturated ester as a common precursor is described. The protocol offers mild reaction conditions, good yields and excellent stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...