An improved kinetic model for cellulose hydrolysis to 5-hydroxymethylfurfural using the solid SO42−/Ti-MCM-41 catalyst
Abstract
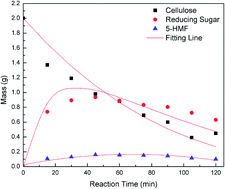
5-Hydroxymethylfurfural (5-HMF) which can be produced from cellulose is considered as one of the promising green platform chemicals. In this work, the conversion of cellulose to 5-HMF was carried out through hydrolysis in a batch reactor using the SO42−/Ti-MCM-41 catalyst at a temperature of 463–503 K. Two analytical techniques including the 3,5-dinitrosalicylic acid (DNS) method and high performance liquid chromatography (HPLC) were employed to track the time evolution of the main reagents, i.e., cellulose, 5-HMF and reducing sugar. Based on the typical kinetic experiments for the cellulose hydrolysis, a comprehensive hydrolysis mechanism was introduced to describe the heterogeneous hydrolysis of cellulose to 5-HMF. A corresponding kinetic model was proposed on the basis of the hydrolysis mechanism and the mass balance law. The results show that the predicted data obtained via the suggested model agree well with the experimental results. In addition, some data from the open reports were referred for further model verification in this work. The kinetic model suggested in this work is a mechanism model for the heterogeneous catalytic cellulose hydrolysis process, which is different from the previous first-order kinetic model.

 Please wait while we load your content...
Please wait while we load your content...