Efficient hydrogenation of imines over Fe and ZnO powder in a self-neutralizing acidic CO2–H2O system†
Abstract
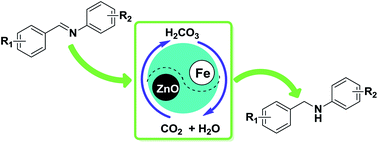
An environmentally benign process was developed for the reduction of imines with iron and zinc oxide-promoted in the reversible CO2–H2O system without any acid additive or organic solvent. H2O is thought to behave as the terminal hydrogen source. The reaction system could be inherently neutralized by the ready removal of CO2, thus resulting in facile post-processing and disposal without waste. The highly flexible and chemo-selective reduction protocol affords the corresponding amines with other intact reducible substituents in good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...