A quantum chemical study on the mechanism and energetics of the direct esterification, thioesterification and amidation of 1-hydroxy-3-methyl-3-phospholene 1-oxide
Abstract
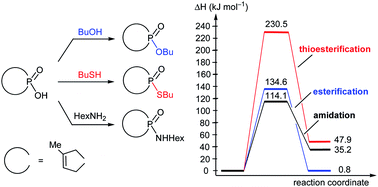
The direct esterification of 1-hydroxy-3-methyl-3-phospholene 1-oxide (1) with 1-butanol is an interesting model, as it fails to occur upon conventional heating, but does, however, take place upon microwave (MW) irradiation. To be able to explain this phenomenon, high level quantum chemical calculations were carried out, considering all relevant intermolecular interactions and applying the explicit and implicit solvent model. The precise energetics (thermodynamic and kinetic data) were in accordance with our experimental observations, and on this basis, we could explain the beneficial effect of MW irradiation. The direct thioesterification and amidation of hydroxy-phospholene oxide 1, occurring only with incomplete conversions under MW irradiation, were also evaluated.

 Please wait while we load your content...
Please wait while we load your content...