Chemical synthesis of nanostructured Cu2Se with high thermoelectric performance†
Abstract
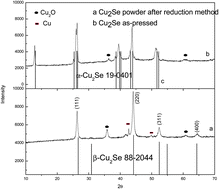
Two simplified chemical processes, a co-precipitation and a reduction method, were developed to synthesize pure Cu2Se nanoparticles. The XRD results indicate that the co-precipitation method is a better way to synthesize high-purity Cu2Se in comparison to the reduction method, where a small amount of Cu2O and Cu are found. Scanning electron microscopy reveals that Cu2Se powder synthesized by the reduction method possesses a morphology dominated by irregular hexagonal nanoplates and spherical nanoparticles; however, only nanoplates are observed in powder prepared by the co-precipitation method. The effect of the two synthetic methods on the thermoelectric properties of Cu2Se, in the temperature range of 300–1000 K, was investigated. The results indicate that the co-precipitation method subsequently combined with a hot-press process, is an effective way to improve Cu2Se thermoelectric performance compared to the reduction method together with hot-pressing. The largest figure of merit, ZT, for Cu2Se fabricated by the co-precipitation method is 1.35, which is 40% larger than that of Cu2Se fabricated by the reduction method (ZTmax = 0.97) due to its good power factor at high temperatures.

 Please wait while we load your content...
Please wait while we load your content...