Clay films with variable metal ions and self-assembled silicate layer-void nanostructures
Abstract
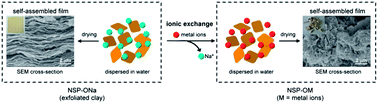
The naturally occurring silicate clays with sodium counter-ions were exfoliated into nanoscale silicate platelets (NSP-ONa) and subsequently exchanged with various metal ions including lithium(I), potassium(I), magnesium(II), calcium(II), and aluminum(III) to afford silicate platelets with the corresponding metal counter-ions (NSP-OM, M = metal counter-ion). In solution, their ionic properties were characterized by measuring zeta potentials over pH to reveal the electrokinetic shifting from −50 mV to −5 mV. The gelling behavior was shown to be low at a concentration of 4 wt% for M = monovalent Na+, K+, and Li+; and 7 wt% for M = multivalent Mg2+, Ca2+, and Al3+. Under the process of solution coating and evaporating, the silicate platelets self-assembled into thin films of 20–100 μm in thickness with the exception of NSP-OAl. The regularity of the clay's self-assembled nanostructure increases as the valence of the metal counter-ion decreases. The nanostructures of the silicate films were further characterized by wide angle X-ray diffraction, density/void measurement, and scanning electron microscopy. These findings of NSP-OM and their self-assembled nanostructures formation were for the first time recorded and suggested for new film fabrications by bottom-up technology.

 Please wait while we load your content...
Please wait while we load your content...